Study Details
Study Design
Prospective, randomized, 1:1, controlled, multi-centre, angiographically documented three-vessel disease (3VD) open-label study
Schematic
- Screening/Eligibility → de-novo 3-vessel CAD.
- Randomization 1:1 → Supraflex Cruz vs Synergy.
- Index PCI → contemporary best-practice techniques permitted.
- Follow-up → Day 30, Month 12 (POCE), Month 24 (VOCE).
- Endpoints → POCE at 12m (non-inferiority); VOCE per-vessel at 24m (superiority).
Definition
- POCE (Patient-Oriented Composite Endpoint): all-cause death, any MI, any stroke, any revascularization.
- VOCE (Vessel-Oriented Composite Endpoint): vessel-related cardiac death, vessel-related MI, target-vessel revascularization.
- QFR: Quantitative Flow Ratio
- CTO: Chronic Total Occlusion
- OMT: Optimal Medical Therapy
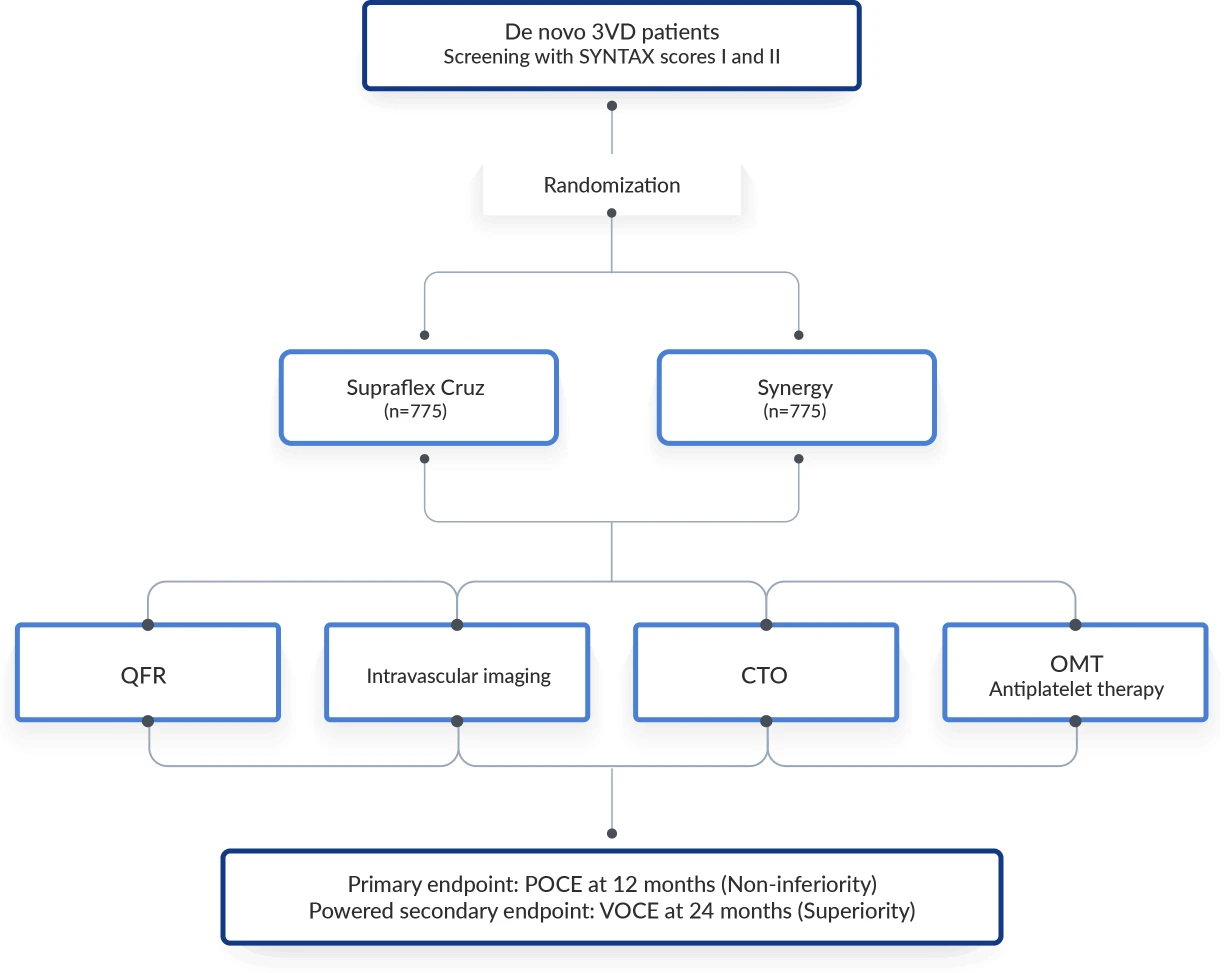
Abbreviation: POCE: Patient-Oriented Composite Endpoint, VOCE: Vessel-Oriented Composite Endpoint, QFR: Quantitative Flow Ratio, CTO: Chronic Total Occlusion, OMT: Optimal Medical Therapy